
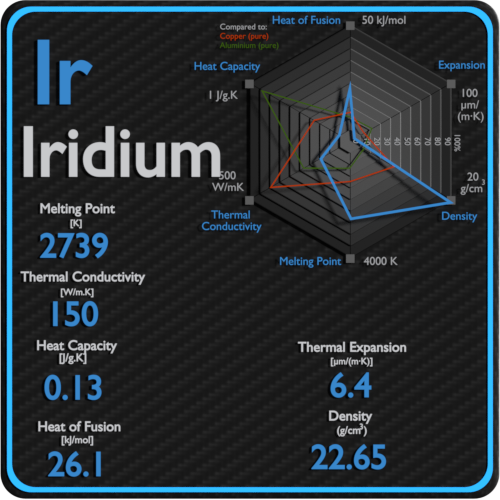
Iridium is found in meteorites in much higher abundance than in the Earth's crust. Iridium radioisotopes are used in some radioisotope thermoelectric generators. Iridium metal is employed when high corrosion resistance at high temperatures is needed, as in high-performance spark plugs, crucibles for recrystallization of semiconductors at high temperatures, and electrodes for the production of chlorine in the chloralkali process. The most important iridium compounds in use are the salts and acids it forms with chlorine, though iridium also forms a number of organometallic compounds used in industrial catalysis, and in research. 191Ir and 193Ir are the only two naturally occurring isotopes of iridium, as well as the only stable isotopes the latter is the more abundant. Iridium is one of the rarest elements in Earth's crust, with annual production and consumption of only 3 tonnes (6.6 thousand pounds). Smithson Tennant, the primary discoverer, named it after the Greek goddess Iris, personification of the rainbow, because of the striking and diverse colors of its salts. Iridium was discovered in 1803 among insoluble impurities in natural platinum. Although only certain molten salts and halogens are corrosive to solid iridium, finely divided iridium dust is much more reactive and can be flammable. It is the most corrosion-resistant metal, even at temperatures as high as 2,000 ☌ (3,630 ☏). A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of 22.56 g/cm 3 (0.815 lb/cu in) as defined by experimental X-ray crystallography. Iridium – Properties Element Iridium Atomic Number 77 Symbol Ir Element Category Transition Metal Phase at STP Solid Atomic Mass 192.217 Density at STP 22.65 Electron Configuration 4f14 5d7 6s2 Possible Oxidation States +3,4 Electron Affinity 151 Electronegativity 2.2 1st Ionization Energy 9.1 Year of Discovery 1803 Discoverer Tennant, Smithson Thermal properties Melting Point 2410 Boiling Point 4130 Thermal Conductivity 150 Specific Heat 0.13 Heat of Fusion 26.Iridium is a chemical element with the symbol Ir and atomic number 77. The melting point is the temperature at which the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. At some point, the amplitude of vibration becomes so large that the atoms start to invade the space of their nearest neighbors and disturb them, and the melting process initiates. As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy. The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as common window glass), and are typically low in energy. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. The first theory explaining the mechanism of melting in bulk was proposed by Lindemann, who used the vibration of atoms in the crystal to explain the melting transition. When considered as the temperature of the reverse change from liquid to solid, it is called the freezing point or crystallization point. The melting point of a substance depends on pressure and is usually specified at standard pressure. Adding heat will convert the solid into a liquid with no temperature change. In thermodynamics, the melting point defines a condition where the solid and liquid can exist in equilibrium. When considered as the temperature of the reverse change from vapor to liquid, it is called the condensation point.
The pressure at which vaporization (boiling) starts to occur for a given temperature is called the saturation pressure. The temperature at which vaporization (boiling) starts to occur for a given pressure is called the saturation temperature or boiling point. In thermodynamics, saturationdefines a condition in which a mixture of vapor and liquid can exist together at a given temperature and pressure. Note that these points are associated with the standard atmospheric pressure. Iridium – Melting Point and Boiling Point


 0 kommentar(er)
0 kommentar(er)
